Analytical Center Research: Dilution Primer
PERCENT, PPM, PPB, PPT
Suppose you were asked you to make one hundred milliliters of a 25 parts per billion (ppb) solution from a five hundred parts per million (ppm) standard. Don't panic! If you can do this off the top of your head you are the exception to the rule. The object of this exercise is to get you to a point where you can easily and simply perform serial dilutions. One of the problems with performing dilutions is that there is no handy dandy, all-purpose formula you can easily refer to. The strategy you use is pretty much left up to you. It is often dependent upon the volume of your available receptacles, pipettes, and the concentration of your initial and final solutions.
CONCENTRATIONS
The most crucial fact to understand is that percent, ppm, and ppb are all concentrations. Since we are dealing with solutions (liquids) in this case, the term concentration, simply says that there is a certain amount of solid "stuff" dissolved in a certain amount of liquid.
DEFINITIONS
These following definitions will form the absolute foundation for serial diluting.
MEMORIZE THESE!!!!
a. A ppm is equal to 1 ug per milliliter
b. A ppb is equal to 1 ng per milliter
c. 1000 ppb equals 1 ppm
d. 1000 ng equals 1 ug
PRACTICAL DEFINITION OF PPB CONCENTRATION
What exactly does it mean when we say that a solution has a concentration of 1 ppb? Well, the term 1ppb should say to you that in this vessel of liquid, for every milliliter of liquid I take out of it , there will be 1ng of "stuff" dissolved in it.
OK. With the definitions under our belts let's try that dilution we mentioned at the beginning of the article.
From a 500 ppb standard lets make 100 mls of a 25 ppb solution. First we need a practical method to approach this problem. You must first ask yourself: How much "stuff" do I need? To find the answer to this we just look at what we were asked for. Now remember our practical definition: in a 1 ppb solution, for every milliliter, there is 1ng of "stuff" in that ml . That is to say, every time we take 1ml of the solution out of its container, 1 ng of "stuff" will be in that 1 ml . Therefore, for a 25 ppb solution we would have 25 ng of "stuff" in 1 ml of liquid.. But we don't need 1ml of a 25 ppb solution; we need 100 mls. So in total we need (100 x 25ng) or 2500 ng of stuff. Now since we know how much stuff we need where do we get it from? We get it from the bottle of 500ppm solution that has been provided to us. Using our practical definition again we know that for every ml of solution we take out of this bottle there will be 500ug of stuff in it. One ml of the 500 ppm solution has 500 ug or by the memorized definitions 1ug = 1000ng therefore 500ug = 500,000ng. Great! That's more than enough. We can easily get our 2500 ng from this. But we only need 2500 ng. (This is where it gets a little tricky and you have to decide what math you will use to figure out how to get your 2500 ng from the 500,000ng. You can use fractions or percentages or proportions. It's up to you. ) We only need that fraction of the ml that will give you 2500ng or 2500ng/500,000ng of a ml. This is equal to 1/200 of a ml of the 500 ppm solution. We then pipette out 1/200 of a ml of the 500ppm solution, place it in a 100 ml volumetric flask, and fill to the mark. (In real world dilutions always try to make the volume, of whatever is being diluted, no less than 1ml)
Congratulations. You have completed your first dilution.
MAKING A SOLUTION FROM A COMPOUND
Suppose you were asked to perform the previous dilution but with a slight twist. Suppose you were asked to make one hundred milliliters of a 25 parts per billion (ppb) solution of lead from a five hundred parts per million (ppm) lead standard but instead of being given the 500 ppm Pb standard the instructor hands you a bottle of lead oxide, PbO. What do you do? Well the first thing you need to know is how much lead is in this lead oxide. First find the formula weight of lead oxide or PbO. Calculate it by simply adding the molecular weight of all the elements in the compound. The formula weight for lead is about 233 grams 207 grams of which is lead and 16 grams of which is oxygen. So we then set up a proportion. You say for every 233 grams of PbO there are 207 grams is Pb therefore for every x grams of PbO there will be y grams of Pb in it . It should look something like this:
![]()
So now you have a way of figuring out how much lead you can get out of the lead oxide. Now you have to figure out how much lead you need to make your 500 ppm lead standard. Again, recall your definition. For every ml of standard there will be 500 ug of lead dissolved in it. Lets say you decide to make 100 mls of the standard so therefore you will need 50,000 ug ,or 50 mg, or .05 grams of lead. So we plug this into the proportion
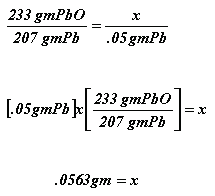
We now simply weigh out .0563 grams of the PbO into a 100 ml volumetric flask and bring it up to the mark with water. Congratulations! You have made your standard 500 ppm solution of lead from PbO and can now carry out the previous dilution.
The contents of this page is written by Willie Johnson and designed by Dhawal Shah, if you need any more information please contact either one of us.